Electrode for Producing High-Efficiency Green Water Based on Nano-Disc Ternary Tungsten Cobalt Boron
- admin
- 2022-08-08
- 975
Professor Jihoon Lee's (Department Of Electronics) Research Team Developed an Electrode for Producing High-Efficiency Green Water Based on Nano-Disc Ternary Tungsten Cobalt Boron (W3cob3)
- JCR ‘Energy and Fuel’ category Q1, IF: 9.257 published in scientific journals
- Using the nano-disc-shaped structure with greatly improved surface area, it is possible to significantly improve hydrogen generation reaction (HER) and oxygen generation reaction (OER) properties and produce ultrapure green hydrogen -

The research team led by Professor Jihoon Lee of the Department of Electronic Engineering at the Department of Electronic Engineering and Ph.D. researcher Ahasan (first author) succeeded in developing a ternary tungsten cobalt boron (W3CoB3)-based nano-disc high-efficiency electrode for green hydrogen production for the first time in the world. The W3CoB3 electrode showed low overpotentials of 84 and 271 mV for HER and OER at 60 mA/cm2 at 1M KOH in the three-electrode system. In addition, the W3CoB3 electrode showed 1.52V at 1M KOH by the two-electrode configuration, so the W3CoB3 electrode was evaluated as one of the best transition metal-based compound electrodes for water decomposition. In the two-electrode system 1M PBS, the RuO2 and W3CoB3 electrodes showed similar OER performance. The electron supply to the transition metal atoms was promoted through the optimal integration of boron atoms, which served to tune the reaction kinetics to obtain improved reaction rates.
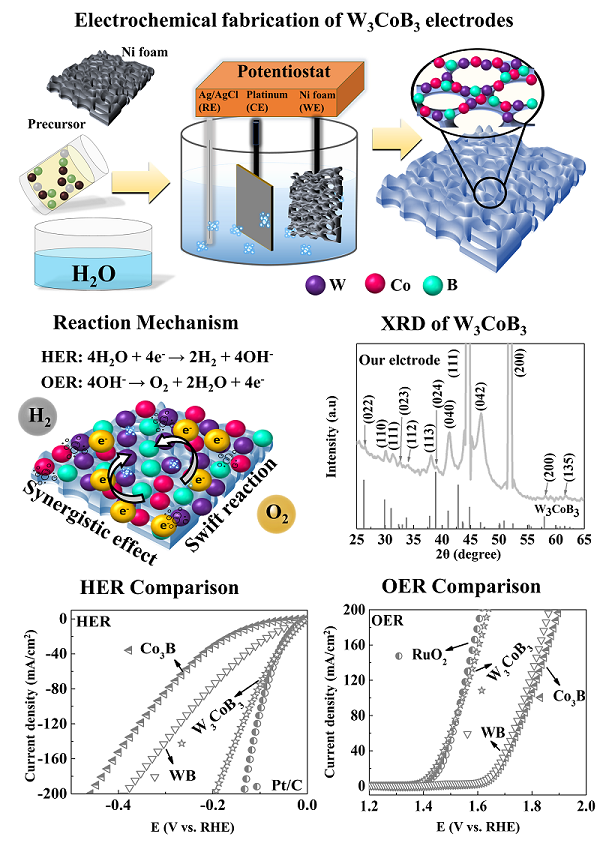
In this study, the design of the nanodisc-shaped ternary W3CoB3 electrode was thoroughly investigated by changes in electrochemical fabrication parameters such as H2SO4 concentration, boron concentration, Co-W concentration, deposition duration, and heat treatment, as shown in the figure. Through systematic optimization, the best nanodisc-shaped W3CoB3 electrode showed significantly better performance than binary Co3B and WB for both OER and HER, as shown in the figure. The nanodisc ternary W3CoB3 catalyst exhibited overpotentials of 84 and 271 mV for HER and OER at 60 mA/cm2, which were significantly improved by the formation of spherical microclusters composed of vertical circular nanoplates with boron with ECSA. was produced due to the significantly improved electrocatalytic activity by the incorporation of The boron-based ternary W3CoB3 electrode by electrochemical fabrication could provide an attractive route to the development of low-cost, high-efficiency, high-performance electrocatalysts that can meet the demand for the green hydrogen economy.
The researchers of this study are Md Ahasan Habib (Ph.D., Kwangwoon University), Rutuja Mandavkar (Ph.D., Kwangwoon University), and Shalmali Burse (Ph.D., Kwangwoon University), Shusen Lin (Ph.D., Kwangwoon University), and Rakesh Kulkarni (Ph.D.) Course, Kwangwoon University), Chandrashekhar S Patil (Ph.D., Kwangwoon University), Jae-hun Jeong (Research Professor, Kwangwoon University-corresponding author), and Jihoon Lee (Professor, Kwangwoon University-corresponding author). Student Md Ahasan Habib entered Kwangwoon University's Ph.D. program in electronics in 2021 and is currently a researcher in the second year of the doctoral program. He has participated in several joint studies to date and has published several co-author papers. First, he was happy that the first author's thesis was published in an excellent journal, and he expressed his ambition to continue his research at an excellent institution after obtaining his doctorate.
Meanwhile, this research was carried out with the support of Kwangwoon University and the BK-21 research institute project promoted by the National Research Foundation of Korea and the Ministry of Education. It was published in the online edition of May 2022 under the title “Design of boron-based ternary W3CoB3 electrocatalyst for the improved HER and OER performances”.
Web link: https://doi.org/10.1016/j.mtener.2022.101021