Prof. Jang Min's Research Team: Eco-Friendly Photo-Piezoelectric Catalyst Synthesis Method
- admin
- 2022-07-14
- 2825
Prof. Jang Min's Research Team
Developed an Eco-Friendly Photo-Piezoelectric Catalyst Synthesis Method
Capable of Controlling Charge Transfer for Selective and High-Efficiency Generation of H₂O₂, a Clean Energy Source and Versatile Oxidizer.
- Published in scientific journal “Applied Catalysis B: Environmental” (JCR IF 24.319, JCR Rank: 1.85%) -
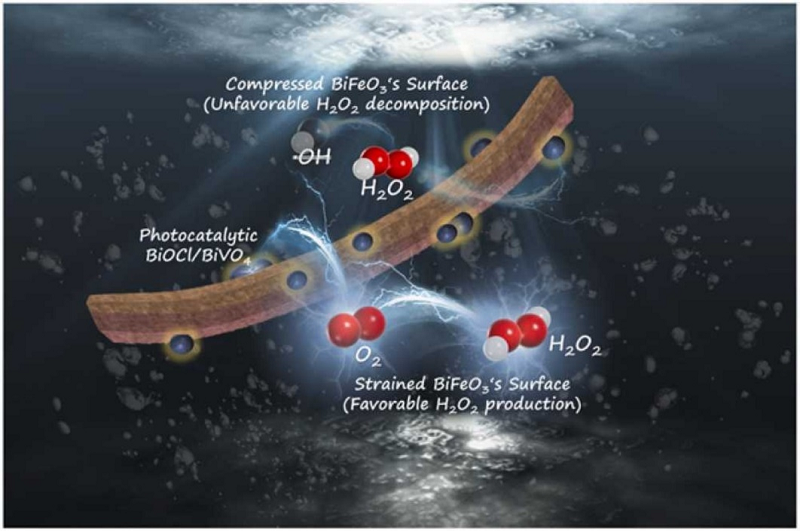
< A photo-piezoelectric catalytic reaction mechanism with controllable charge transfer for the selective generation of H₂O₂ >

(left to right) Dr. Kien Tiek Wong, Research Professor Choong Choe Earn, Professor Eunha Choi, Professor Min Jang
Kien Tiek Wong, a postdoctoral researcher (first author) and Choong Choe Earn Research Professor from the research team of Professor Min Jang (corresponding author) at the Department of Environmental Engineering of Kwangwoon University, and Professor Eunha Choi's team at the Plasma Bio-Center have developed a piezoelectric catalyst preparation synthesis method. They used BVO/BFO, a composite in which nano bismuth oxychloride/bismuth vanadate (BiOCl/BiVO, BVO) is homogeneously distributed in BiFeO3 nanorod (BFO), and photo- and H₂O₂ that can produce significantly higher H₂O₂ compared to catalysts that have been studied in the past.
Hydrogen peroxide (H₂O₂) is a versatile oxidizing agent used in a variety of applications including water treatment, chemical manufacturing, and medicine owing to its high oxidation potential and benign byproducts. Additionally, H₂O₂ is more convenient to transport and store compared with compressed H₂ gas, which is also an environmentally friendly energy source. Therefore, H₂O₂ has recently generated considerable interests as a potential alternative to fossil fuels. To meet global demand without entirely relying on fossil fuels, the energy source must have an output potential comparable to that of a hydrogen fuel cell (1.23 V). The H₂O₂ fuel cell (1.09 V) can meet this requirement as an alternative fuel source. Currently, there is only one main method of the oxidation of anthraquinone for the industrial production of H₂O₂. However, there are disadvantages in the method: high energy costs, toxic by-products, chemicals and expensive precious metal catalysts, and especially excessive CO₂ emissions. Therefore, an environmentally friendly process is needed to replace it.
In general, photo-induced charge carriers are separated into electrons and positive holes through irradiation with a light source in a photocatalyst. The separated electrons and holes can react with oxygen and H₂O in water, respectively, to generate various radicals. In addition, in the case of a piezoelectric catalyst, an electrical reaction occurs under the influence of pressure. In this study, the photo-piezoelectric catalyst BVO/BFO composite created with the synthetic method by the research team manipulated the flow of electrons through the band potential and modified in the photo-piezoelectric process. It was confirmed that changing the interfacial charge transfer inhibited the dissociation of the O-O bond of H₂O₂ and promoted selective H₂O₂ production. This study showed higher H₂O₂ production compared to most other previously published studies. Thus, it suggested the possibility of a photo-piezoelectric catalyst for efficient H₂O₂ production.
This study was carried out as a basic science research support project (No. 2020R1F1A1075839) supported by the National Research Foundation of Korea Ministry of Education and by the Ministry of Science and ICT. The research results were published under the title of “Interfacial Schottky junctions modulated by photo-piezoelectric band bending to govern charge carrier migration for selective H₂O₂ generation” in the online edition of Applied Catalysis B: Environmental (IF: 24.319, JCR rank: 1.85%), the No. 1 scientific journal in the field of environmental engineering on October 15, 2022.
Web link: https://www.sciencedirect.com/science/article/pii/S0926337322005227